It has been proposed that Canadian nuclear fuel waste be disposed of 500-1000 m deep in either granitic rock or sedimentary clay deposits. Within the multi-barrier system proposed, the fuel wastes would be sealed in a metallic container, which, in a sedimentary clay environment, could be a single thick-walled design fabricated from carbon steel.

Raman analyses of several spots on steel surfaces exposed to either high (4.77 M) or low (0.10 M) chloride environments were used to identify the corrosion products. The peak from 650-750 cm-1 (Figure 1) was deconvoluted into the A1g modes for the symmetrical stretches of the magnetite (Fe3O4) (667 cm-1) and maghemite (γ-Fe2O3) (710 cm-1) peak contributions. Over 14 days of exposure, the height and area of the magnetite peak increases dramatically, indicating a conversion of initially formed maghemite to magnetite in the corrosion product layer.
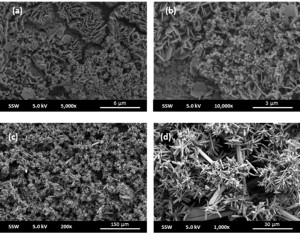
SEM analysis of the surface features shows that the films formed in low and high chloride environments do not differ significantly in morphology (Figure 2 (a) and (b)). The sample exposed to simulated groundwater solution for 56 days is shown to be covered in a network of aragonite (CaCO3) and gypsum (CaSO4) crystals (Figure 2 (c) and (d)).
By Shannon Hill, Department of Chemistry and Surface Science Western

