The proposed Canadian container for the disposal of spent nuclear fuel consists of a dual wall comprised of an outer shell of copper over an inner low alloy steel vessel. Because of manufacturing and fabrication challenges for thick-walled containers, a thin-walled alternative design is being developed. Their fabrication involves coating copper directly on the surface of the steel vessel to produce a single-walled container capable of withstanding the required mechanical loading and with a sufficient corrosion resistance. Two coating methods are currently being considered: cold-spray coating and electrodeposition.
To simulate long-term corrosion, electrochemically accelerated dissolution experiments are being conducted to determine whether the different grain structures, morphologies and strain distributions in the copper-coated specimens will have any significant influence on their susceptibility to localized/intergranular corrosion.
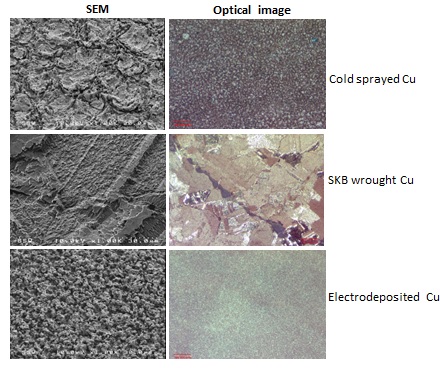
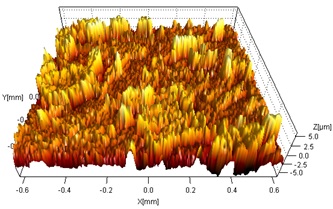
The electrochemically-treated specimens were analyzed by SEM/EDX spectroscopy, to determine the structure, morphology, and composition of the corrosion products. The images in Figure A show the morphology of the electrochemically-dissolved surface for both types of coatings compared to that obtained by similarly accelerating the dissolution of wrought copper. The etching patterns are different for the three specimens, suggesting that the type and location of corrosion damage will vary with the type of coating. Figure B shows a profilometry profile recorded on the wrought copper. The extent of surface roughness is an indication of the depth to which corrosion might penetrate.
By Dr. Raheleh Partovi-Nia, Department of Chemistry, University of Western Ontario

